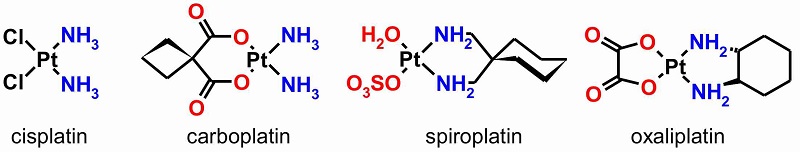
Cisplatin is a major anti-cancer drug, whose effect is based on its binding to DNA. Since its discovery, intense research efforts have been devoted to modifications of cisplatin that lead to second-generation drugs as spiroplatin, carboplatin and oxaliplatin. However, these modifications are based on the same leitmotif with variations in the ligands. Thus, these anti-cancer drugs work in the same way by binding to the nucleobases of DNA.
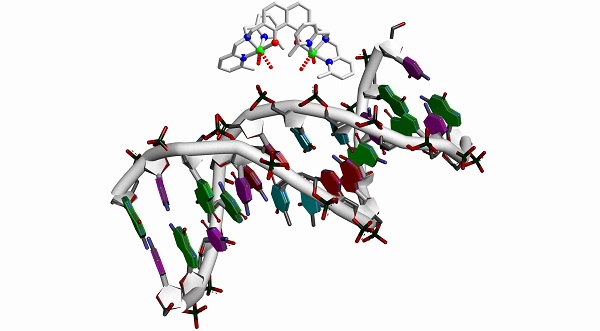
Inspired by the success of cisplatin, we intended to create a new class of anti-cancer drugs that works by binding to the phosphodiester groups of DNA. Our intention was to design a transition metal complex that binds preferentially at the phosphates of DNA and not the nucleobases in order to obtain a new class of potentially cytotoxic compounds. Although the nucleobases are regarded as better ligands for transition metal ions than the phosphates of DNA, the metal ions of nucleases and phosphatases and their biomimetic model complexes bind specifically at the phosphates due to molecular recognition. In our design approach, the molecular recognition for the phosphates of the DNA is based on two pre-oriented metal ions fixed at the distance of 6-7 Å, which is the distance of two neighboring phosphates in the DNA backbone, by a rigid spacer. This should enhance the binding affinity by the multivalence principle. Moreover, bulky pendant arms should suppress binding to the nucleobases of the DNA, which are less exposed, by sterical hindrance.
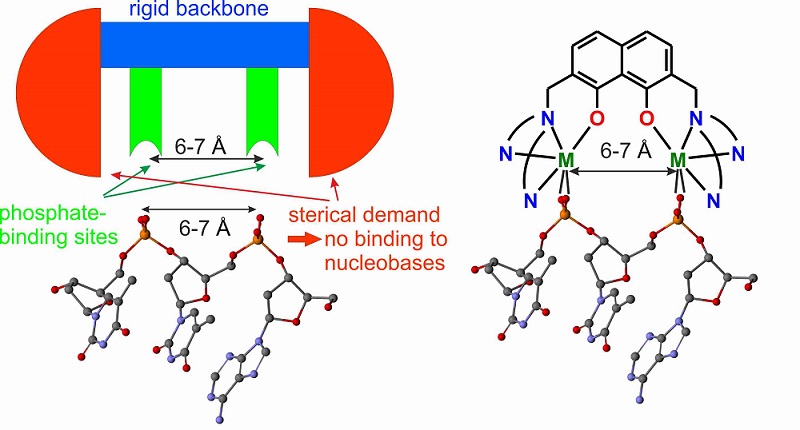
These design principles were translated into a family of dinuclear complexes based on 2,7-disubstituted 1,8-naphthalenediol ligands. Based on this design, we have established a synthetic route to 2,7-disubstituted 1,8-naphthalenediol ligands and synthesized the dinuclear copper(II) complex shown below.
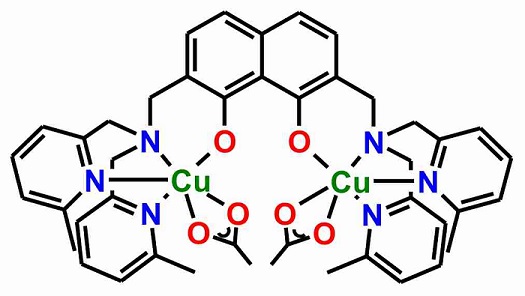
Several independent experiments at the single-molecule level and ensemble experiments in solution show a strong irreversible binding to DNA. This irreversible binding to DNA leads to a blocking of DNA synthesis as studied by polymerase chain-reactions. Moreover, this complex kills HeLa cancer cells more efficiently at low concentrations than cisplatin.
Related Publications
J. Simon, A. Stammler, J. Oldengott, H. Bögge, and T. Glaser
"Proof of Phosphate Diester Binding Ability of Cytotoxic DNA-Binding Complexes"
Inorg. Chem., 2020, 14615-14619.- S. Schwarzbich, C. Horstmann née Gruschka, J. Simon, L. Siebe, A. Moreth, C. Wiegand, a. Lavrentieva, T. Scheper, A. Stammler, H. Bögge, G. Fischer von Mollard, and T. Glaser
"Stronger Cytotoxicity for Cancer Cells Than for Fast Proliferating Human Stem Cells by Rationally Designed Dinuclear Complexes"
Inorg. Chem., 2020, 14464-14477. T. Glaser, G. Fischer von Mollard, D. Anselmetti
"Rational Design of Dinuclear Complexes Binding at Two Neighboring Phosphate Esters of DNA"
Inorg. Chim. Acta, 2016, 452, 62-72.T. Jany, C. Horstmann, H. Bögge, A. Stammler and T. Glaser
"A Series of Dinuclear Complexes with a Flexible Naphtalene-Spacer and MOM-Cleavage by Pre-coordinated Lewis Acids"
Z. Anorg. Allg. Chem., 2015, 641,2157-2168.T. Jany, A. Moreth, C. Gruschka, A. Sischka, A. Spiering, M. Dieding, Y. Wang, S. H. Samo, A. Stammler, H. Bögge, G. Fischer von Mollard, D. Anselmetti, and T. Glaser
"Rational Design of a Cytotoxic Dinuclear Cu2 Complex That Binds by Molecular Recognition at Two Neighboring Phosphates of the DNA Backbone"
Inorg. Chem., 2015, 54, 2679-2690.T. Glaser, I. Liratzis, R. Fröhlich, and T. Weyhermüller
"A Trinucleating Ligand Based on 1,8-Naphthalenediol: Synthesis, Structural and Magnetic Properties of a Linear CuIICuIICuII Complex"
Chem. Comm. 2007, 356-358.T. Glaser and I. Liratzis
"A Streamlined Synthesis for 2,7-Diformyl-1,8-Naphthalenediol"
Synlett 2004, 735-737.